Sodium sulphide (sulfide) is a chemical compound with the formula Na2S. It is commonly used in the paper and pulp industry as well as in water treatment to remove heavy metals.
| IUPAC Name | Disodium sulfide |
| Molecular Formula | Na2S |
| CAS Number | 1313-82-2 |
| Synonyms | Sodium sulfide, sodium monosulfide, disodium sulfide, sodium sulphide anhydrous, sodium sulphide nonahydrate, sodium sulfuret |
| InChI | InChI=1S/2Na.S/q2*+1;-2 |
Sodium sulfide molar mass
Sodium sulfide has a molar mass of 78.045 g/mol. This means that one mole of sodium sulfide contains 78.045 grams of the compound. The molar mass is a useful quantity for calculating the amount of a substance needed for a reaction, as well as for determining the molecular weight of a compound.
Sodium sulfide boiling point
Sodium sulfide has a relatively high boiling point of >1,180°C (2,156°F). This means that it requires a significant amount of energy to convert the solid form of sodium sulfide to its gaseous form. The high boiling point also indicates that sodium sulfide is a stable compound at high temperatures.
Sodium sulfide melting point
Sodium sulfide has a melting point of 1,180°C (2,156°F). This means that it requires a significant amount of energy to convert the solid form of sodium sulfide to its liquid form. The high melting point of sodium sulfide also indicates that it is a stable compound at high temperatures.
Sodium sulfide density g/ml
The density of sodium sulfide varies depending on its form. The anhydrous form of sodium sulfide has a density of 1.856 g/mL, while the nonahydrate form has a density of 1.58 g/mL. The density of a substance is the mass per unit volume, and it is a useful quantity for determining the concentration of a solution.
Sodium sulfide molecular weight
The molecular weight of sodium sulfide is 78.045 g/mol. This means that one mole of sodium sulfide contains 6.022 x 10^23 molecules of the compound. The molecular weight is a useful quantity for calculating the amount of a substance needed for a reaction, as well as for determining the molar mass of a compound.
Sodium sulfide Structure
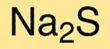
Sodium sulfide has a crystalline structure in its anhydrous form. The compound forms colorless crystals that are highly soluble in water. The structure of the compound is composed of sodium cations (Na+) and sulfide anions (S2-). The compound has a strong ionic bond between the sodium and sulfide ions.
Sodium sulfide formula
The chemical formula for sodium sulfide is Na2S. This means that each molecule of sodium sulfide contains two sodium cations and one sulfide anion. The formula is useful for understanding the chemical composition of the compound and for determining its properties.
| Appearance | Yellow to yellow-brown solid, deliquescent |
| Specific Gravity | 1.856 (anhydrous), 1.58 (nonahydrate) |
| Color | Colorless to yellow |
| Odor | Rotten egg-like odor |
| Molar Mass | 78.045 g/mol |
| Density | 1.856 g/mL (anhydrous), 1.58 g/mL (nonahydrate) |
| Melting Point | 1,180°C (2,156°F) |
| Boiling Point | >1,180°C (2,156°F) |
| Flash Point | Not applicable |
| Water Solubility | Highly soluble |
| Solubility | Soluble in ethanol, glycerol |
| Vapor Pressure | Not applicable |
| Vapor Density | Not applicable |
| pKa | Not applicable |
| pH | Alkaline, pH ~12 |
Sodium Sulphide Safety and Hazards
Sodium sulphide can pose several safety hazards. It is highly reactive with water and can cause severe skin and eye irritation upon contact. Inhaling the compound can lead to respiratory tract irritation and can cause nausea, vomiting, and diarrhea if ingested. The compound is also highly flammable and can react violently with oxidizing agents. It is important to handle sodium sulphide with care and wear appropriate protective equipment, such as gloves and goggles. Proper ventilation should also be ensured when handling the compound. In case of exposure, immediate medical attention should be sought.
| Hazard Symbols | Corrosive, Toxic |
| Safety Description | Do not breathe dust/fumes/gas/mist/vapours/spray. Wear protective gloves/protective clothing/eye protection/face protection. IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. Immediately call a POISON CENTER or doctor/physician. |
| UN Ids | UN1849 |
| HS Code | 2830.10.00 |
| Hazard Class | 8 |
| Packing Group | II |
| Toxicity | Highly toxic; may cause severe skin and eye irritation, respiratory tract irritation, nausea, vomiting, and diarrhea if ingested. May be fatal if swallowed. |
Sodium Sulphide Synthesis Methods
Several methods exist for synthesizing sodium sulfide:
One common method involves reacting sodium hydroxide (NaOH) with sulfur (S) in a furnace at high temperatures to produce sodium sulfide (Na2S) and water (H2O).
Another method involves reacting sulfur dioxide (SO2) with sodium sulfide (Na2S) to form sodium thiosulfate (Na2S2O3) and then further reacting it with sodium hydroxide (NaOH) to form sodium sulfide (Na2S) and water (H2O).
A third method involves the reduction of sodium sulfate (Na2SO4) with carbon in the presence of charcoal at high temperatures to produce sodium sulfide (Na2S), carbon dioxide (CO2), and carbon monoxide (CO).
Another method involves reacting sodium carbonate (Na2CO3) with sulfur (S) in the presence of charcoal at high temperatures to produce sodium sulfide (Na2S), carbon dioxide (CO2), and carbon monoxide (CO).
To synthesize sodium sulfide, one can react sodium sulfide nonahydrate (Na2S.9H2O) with sulfuric acid (H2SO4) to produce sodium sulphate (Na2SO4) and sulfur dioxide (SO2). Then, heat the sodium sulphate with charcoal to produce sodium sulfide (Na2S) and carbon dioxide (CO2).
Overall, there are various methods for synthesizing Sodium sulfide, and the selection of a particular method depends on factors such as the desired purity, quantity, and cost-effectiveness of the product.
Sodium Sulphide Uses
Sodium sulphide serves various industries with a wide range of uses.
The leather industry commonly uses it as a depilatory agent to remove hair and wool from hides and skins.
It finds usage as a sulfiding agent for producing sulfur dyes for textiles and as a reducing agent in manufacturing other chemicals like sodium hydrosulfite.
In the pulp and paper industry, sodium sulphide acts as a bleach aid and helps remove lignin from wood pulp.
It also serves as a flotation agent in mining to separate copper, lead, and zinc ores from their gangue.
In the wastewater treatment industry, sodium sulphide finds use as a reducing agent to remove heavy metals from effluent streams. Additionally, it helps as a dechlorination agent to remove residual chlorine in water treatment processes.
The pharmaceutical industry uses sodium sulphide in producing certain drugs and as a reducing agent in organic chemistry reactions.
Laboratories use it as a reagent in analytical chemistry and as a source of sulfur ions in chemical synthesis.
Overall, Sodium sulphide is a versatile compound with diverse applications in various industries. Its unique chemical properties make it useful as a reducing agent, depilatory agent, flotation agent, and dechlorination agent, among other things.
Questions:
Q: What is the charge on the cation in the ionic compound sodium sulfide?
A: The cation in sodium sulfide is sodium (Na+), which has a charge of +1.
Q: What is the formula for sodium sulfide?
A: The formula for sodium sulfide is Na2S, which represents the ionic compound formed by the combination of one sodium cation (Na+) and one sulfide anion (S2-).
Q: What is the formula for sodium sulfide?
A: The formula for sodium sulfide is Na2S, which represents the ionic compound formed by the combination of one sodium cation (Na+) and one sulfide anion (S2-).
Q: Is sodium sulfide soluble?
A: Sodium sulfide is soluble in polar solvents like water and ethanol, but it is insoluble in non-polar solvents like benzene and chloroform.
Q: Is sodium sulfide soluble in water?
A: Yes, sodium sulfide is highly soluble in water, with a solubility of approximately 50 g/100 mL at room temperature. When sodium sulfide is dissolved in water, it undergoes hydrolysis to form sodium hydroxide and hydrogen sulfide gas.
