Methanol (CH3OH) is a colorless, flammable liquid with a sweet odor. It is used as a solvent, fuel, and antifreeze. It can be toxic if ingested and can cause blindness or death.
| IUPAC Name | Methanol |
| Molecular Formula | CH4O |
| CAS Number | 67-56-1 |
| Synonyms | Methyl alcohol, Wood alcohol, Hydroxymethane, Carbinol, Wood naphtha, Colonial spirit |
| InChI | InChI=1S/CH4O/c1-2/h2H,1H3 |
Note: InChI is a long string that represents the unique structure of a molecule. It may not render properly on some devices.
Methanol Properties
Methanol Molar Mass
The molar mass of Methanol is 32.04 g/mol. It is calculated by summing up the atomic weights of carbon, hydrogen, and oxygen atoms in a single molecule of Methanol. This value is useful for determining the amount of Methanol required to prepare a certain concentration of a solution or for determining the stoichiometry of a chemical reaction.
Methanol Boiling Point
Methanol has a boiling point of 64.7 °C (148.46 °F). This relatively low boiling point makes Methanol useful as a solvent in various industrial applications, including the production of formaldehyde, acetic acid, and methyl methacrylate. However, Methanol’s low boiling point also makes it volatile and flammable, which can pose safety hazards during storage, handling, and transport.
Methanol Melting Point
CH3OH has a melting point of -97.6 °C (-143.68 °F). This means that CH3OH is a liquid at room temperature but can be easily frozen into a solid by cooling it to below its melting point. The low melting point of CH3OH is also useful in certain laboratory applications, such as the preparation of cryogenic solvents for nuclear magnetic resonance spectroscopy.
Methanol Density g/mL
The density of CH3OH is 0.792 g/mL at 20 °C (68 °F). This means that CH3OH is less dense than water and can float on top of it. The density of CH3OH can also be used to calculate the volume of CH3OH needed to obtain a certain mass or concentration of the solution.
Methanol Molecular Weight
The molecular weight of CH3OH is 32.04 g/mol. This value is calculated by adding the atomic weights of carbon, hydrogen, and oxygen atoms in a single molecule of CH3OH. The molecular weight of CH3OH is useful for determining its physical properties, such as boiling point, melting point, and density.
Methanol Structure
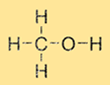
Methanol has a simple structure consisting of a methyl group (CH3) and a hydroxyl group (-OH) attached to a single carbon atom. The molecular formula of Methanol is CH3OH. Methanol is a polar molecule due to the presence of the hydroxyl group, which makes it soluble in polar solvents such as water and ethanol.
Methanol Formula
The chemical formula of Methanol is CH3OH. This means that each Methanol molecule contains one carbon atom, four hydrogen atoms, and one oxygen atom. The formula of Methanol can be used to determine the molecular weight and other physical properties of Methanol. The chemical formula of Methanol is also useful for writing chemical equations and balancing chemical reactions involving Methanol.
| Appearance | Colorless liquid |
| Specific Gravity | 0.792 g/mL at 20°C |
| Color | Colorless |
| Odor | Sweet, pungent |
| Molar Mass | 32.04 g/mol |
| Density | 0.792 g/mL at 20°C |
| Melting Point | -97.6°C |
| Boiling Point | 64.7°C |
| Flash Point | 11.1°C |
| Water Solubility | Miscible |
| Solubility | Miscible with ethanol, ether, acetone, and many organic solvents |
| Vapor Pressure | 13.02 kPa at 20°C |
| Vapor Density | 1.11 (air = 1) |
| pKa | 15.5 |
| pH | 7 (neutral) |
Note: The values listed in this table are approximate and may vary depending on the specific conditions and sources of Methanol.
Methanol Safety and Hazards
CH3OH poses various safety hazards and should be handled with caution. It is a flammable liquid and can ignite if exposed to heat, sparks, or flames. CH3OH vapors can also form explosive mixtures with air. Inhaling CH3OH vapors can cause dizziness, headache, nausea, and other health problems. CH3OH is also toxic and can be absorbed through the skin or ingested, leading to serious health complications, including blindness, coma, and death. It is important to wear protective clothing and handle CH3OH in a well-ventilated area with proper safety equipment, such as goggles, gloves, and a respirator. Spills should be cleaned up immediately and disposed of properly.
| Hazard Symbols | Skull and crossbones, Flammable |
| Safety Description | Keep away from heat/sparks/open flames/hot surfaces. Use explosion-proof electrical/ventilating/lighting equipment. Keep the container tightly closed. Ground/bond container and receiving equipment. Use only non-sparking tools. Take precautionary measures against static discharge. Avoid breathing vapor. Wash thoroughly after handling. Do not eat, drink or smoke when using this product. |
| UN Ids | UN1230 |
| HS Code | 29051100 |
| Hazard Class | 3 |
| Packing Group | II |
| Toxicity | Methanol is toxic and can cause blindness, coma, and death if ingested, inhaled, or absorbed through the skin. It can also cause irritation, redness, and burns upon contact. Exposure to Methanol vapors can cause headaches, dizziness, nausea, and other health problems. Methanol should be handled with extreme caution and proper protective equipment. |
Note: The values listed in this table are approximate and may vary depending on the specific conditions and sources of Methanol. It is important to refer to the relevant safety data sheet and regulations when handling Methanol.
Methanol Synthesis Methods
Direct oxidation of methane, partial oxidation of natural gas, gasification of coal, and synthesis from carbon dioxide and hydrogen all synthesize Methanol.
One of the most common methods for Methanol synthesis is through the catalytic conversion of synthesis gas, which is a mixture of carbon monoxide, carbon dioxide, and hydrogen. This process involves the use of a catalyst, typically copper-based, to facilitate the reaction. The synthesis gas is fed into a reactor containing the catalyst at high temperatures and pressures, resulting in the production of Methanol.
Another method for Methanol synthesis is through the direct oxidation of methane, which involves the use of high temperatures and pressures in the presence of a catalyst such as platinum or palladium. This method is more difficult and expensive than the synthesis gas method, but it is more efficient and can produce higher yields.
The partial oxidation of natural gas is another method for Methanol synthesis, which involves the use of oxygen or air to partially oxidize the natural gas, producing a mixture of carbon monoxide and hydrogen. The mixture is then fed into a reactor containing a catalyst, resulting in the production of Methanol.
The gasification of coal is another method for Methanol synthesis, which involves the conversion of coal into synthesis gas, followed by the same catalytic conversion process as described above.
Carbon capture and utilization convert carbon dioxide emissions from industrial processes into Methanol, using hydrogen as a reducing agent.
Methanol Uses
Methanol has a wide range of uses in various industries, including:
- Fuel: Vehicles, generators, and other industrial equipment can use methanol as fuel
- Solvent: Methanol is an excellent solvent for many substances, including resins, dyes, and oils. Manufacturers use methanol to produce paints, varnishes, and other coatings.
- Chemical intermediate: Used as a raw material for the production of formaldehyde, acetic acid, and other chemicals.
- Antifreeze: Used as an antifreeze agent in automobile cooling systems.
- Deicing agent: Used as a deicing agent for airplane wings and airport runways.
- Cleaner: Used as a cleaning agent in various industrial processes.
- Pharmaceuticals: Used as a solvent in the production of many pharmaceuticals.
- Alternative energy: Investigated as an alternative fuel for fuel cells and as a potential energy storage medium.
- Methanol-to-olefins (MTO): Olefins such as ethylene and propylene derive from converting methanol.
- Methanol-to-gasoline (MTG): A process called MTG converts methanol into gasoline.
Questions:
Q: What is methanol?
A: Methanol, also known as wood alcohol, is a colorless, flammable liquid with a sweet odor. It is the simplest alcohol and has the chemical formula CH3OH.
Q: Is methanol polar or nonpolar?
A: Methanol is a polar molecule. It has a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom, resulting in a dipole moment.
Q: What is methanol used for?
A: Methanol has a wide range of uses in various industries, including as a fuel, solvent, chemical intermediate, antifreeze, deicing agent, cleaner, pharmaceutical solvent, and potential energy storage medium. It is also used in the production of formaldehyde, acetic acid, and other chemicals. Additionally, methanol is converted to olefins such as ethylene and propylene, which are used as raw materials for the production of plastics.
