Caustic potash (KOH) or potassium hydroxide is a highly corrosive alkaline substance used in the production of soaps, detergents, and other chemicals. It reacts violently with acids and can cause severe skin and eye irritation.
| IUPAC Name | Potassium hydroxide |
| Molecular Formula | KOH |
| CAS Number | 1310-58-3 |
| Synonyms | Potash lye, Potassium hydrate, Caustic potash solution, Potassium hydroxide pellets, KOH |
| InChI | InChI=1S/K.1H2O/h;1H2/q+1;/p-1 |
Potassium Hydroxide molar mass
Potassium hydroxide (KOH) has a molar mass of 56.11 g/mol, which is the sum of the atomic weights of potassium (39.10 g/mol) and oxygen (16.00 g/mol) plus one hydrogen (1.01 g/mol) atom. The molar mass of KOH is important in various chemical calculations, including stoichiometry and determining the amount of KOH needed to react with other substances. It is also used to convert between mass, moles, and volume of KOH in different chemical reactions.
Potassium hydroxide formula
The chemical formula of caustic potash is made up of one potassium ion (K+) and one hydroxide ion (OH-), giving it the formula KOH. The formula represents the number and type of atoms that make up a single unit of potassium hydroxide. The formula is used in various chemical calculations, including stoichiometry and determining the amount of potassium hydroxide required for a particular reaction.
Potassium hydroxide boiling point
Potassium hydroxide has a high boiling point of 1,327 °C (2,421 °F). This high boiling point is due to its ionic nature, which requires a significant amount of energy to break the bonds between potassium and hydroxide ions. KOH is used as a strong base and electrolyte in various chemical processes, and its high boiling point makes it suitable for high-temperature applications.
Potassium hydroxide melting point
Potassium hydroxide has a melting point of 360 °C (680 °F). This high melting point is also due to its ionic nature, which requires a significant amount of energy to break the bonds between potassium and hydroxide ions. KOH is commonly used in the production of soaps, detergents, and other chemicals, and its high melting point ensures that it remains in a solid state at room temperature.
Potassium hydroxide density g/ml
Potassium hydroxide has a density of 2.044 g/mL at room temperature. This high density is due to the close packing of KOH molecules in its solid state. The density of KOH is important in determining the volume and mass of KOH required in various chemical reactions. It is also used in determining the concentration of KOH solutions in terms of mass per volume.
Potassium hydroxide structure
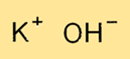
Potassium hydroxide has a crystal structure that consists of potassium ions (K+) and hydroxide ions (OH-) arranged in an orderly fashion. The KOH crystal lattice is a three-dimensional network of ions held together by ionic bonds. The crystal structure of KOH gives it its characteristic physical and chemical properties.
Potassium hydroxide molecular weight
The molecular weight of potassium hydroxide is 56.11 g/mol. This is the sum of the atomic weights of potassium, oxygen, and hydrogen in KOH. The molecular weight of KOH is important in various chemical calculations, including determining the amount of KOH required for a particular chemical reaction.
| Appearance | White or colorless solid |
| Specific Gravity | 2.044 g/mL at 25 °C |
| Color | Colorless |
| Odor | Odorless |
| Molar Mass | 56.11 g/mol |
| Density | 2.044 g/mL at 25 °C |
| Melting Point | 360 °C (680 °F) |
| Boiling Point | 1,327 °C (2,421 °F) |
| Flash Point | Not applicable |
| Water Solubility | Completely soluble in water |
| Solubility | Soluble in ethanol, methanol |
| Vapor Pressure | 0.01 mmHg at 25 °C |
| Vapor Density | Not applicable |
| pKa | 13.5 |
| pH | 13.5 – 14.0 |
Caustic Potash (KOH) Safety and Hazards
Caustic potash (KOH) is a highly corrosive substance that can cause severe burns if it comes into contact with the skin or eyes. It can also be harmful if inhaled or ingested. The substance should be handled with caution, and appropriate personal protective equipment should be worn when handling it. In case of contact with skin or eyes, the affected area should be flushed with water immediately for at least 15 minutes. Caustic potash (KOH) should be stored in a cool, dry, well-ventilated area away from incompatible substances such as acids and oxidizers. Proper labeling and safety information should be provided when handling and transporting the substance.
| Hazard Symbols | Corrosive |
| Safety Description | Causes severe skin burns and eye damage. Harmful if inhaled or ingested. |
| UN Ids | UN1813 |
| HS Code | 2815.12.00 |
| Hazard Class | 8 – Corrosive Substances |
| Packing Group | II |
| Toxicity | Highly toxic |
Caustic Potash (KOH) Synthesis Methods
Caustic potash (KOH) can be synthesized by several methods.
- One common method of synthesizing KOH is through the electrolysis of a potassium chloride (KCl) solution using a membrane cell. In this process, potassium ions migrate to the cathode and undergo reduction to produce potassium metal, which reacts with water to yield KOH and hydrogen gas. Chloride ions migrate to the anode and undergo oxidation to produce chlorine gas.
- Another method of KOH synthesis is the reaction of potassium carbonate (K2CO3) with calcium hydroxide (Ca(OH)2) in a metathesis reaction. The resulting products are CaCO3 and KOH, and the CaCO3 can be filtered out, leaving behind a solution of KOH.
- The reaction between KOH and CO2 yields potassium bicarbonate (KHCO3). Heating potassium bicarbonate (KHCO3) releases carbon dioxide and leaves behind pure KOH.
- The reaction of potassium chloride (KCl) with calcium hydroxide (Ca(OH)2) produces a solution, which upon electrolysis, yields KOH and hydrogen gas.
The choice of method for KOH synthesis depends on factors such as cost, availability of reagents, and desired purity of the final product.
Caustic Potash (KOH) Uses
- Various industries and commercial applications use Caustic potash (KOH) as a strong base in the production of chemicals like potassium carbonate, potassium permanganate, and potassium phosphate.
- Caustic potash (KOH) helps emulsify fats and oils, making it ideal for use in the production of liquid soaps and detergents.
- Farmers use Caustic potash (KOH) as a fertilizer to provide potassium, an essential nutrient for plant growth, and in the production of insecticides and fungicides.
- The manufacture of batteries, especially nickel-metal hydride batteries, uses Caustic potash (KOH) to enhance performance and lifespan.
- In the pharmaceutical industry, Caustic potash (KOH) produces potassium supplements and certain antibiotics.
- Caustic potash (KOH) also plays a role in the production of biodiesel and acts as a catalyst in the production of synthetic rubber.
- Analytical chemistry applications utilize Caustic potash (KOH) in titration of acids and the preparation of standard solutions.
- Caustic potash (KOH) treats various types of waste, such as acidic wastewater and industrial effluents.
Questions:
Q: Is potassium hydroxide a strong base?
A: Yes, potassium hydroxide (KOH) is a strong base.
Q: What is potassium hydroxide?
A: Potassium hydroxide (KOH) is a white, odorless solid that is commonly used as a strong base in various industrial and commercial applications.
Q: Is potassium hydroxide soluble?
A: Yes, potassium hydroxide is highly soluble in water, with a solubility of approximately 121 g/100 mL at room temperature.
Q: What products form from the reaction of sulfuric acid (H2SO4) with potassium hydroxide (KOH)?
A: The reaction of sulfuric acid (H2SO4) with potassium hydroxide (KOH) produces potassium sulfate (K2SO4) and water (H2O).
H2SO4 + 2KOH → K2SO4 + 2H2O
Q: What is the formula for potassium hydroxide?
A: The formula for potassium hydroxide is KOH.
