Benzene or C6H6 is a colorless, highly flammable liquid with a sweet odor. It is used in the production of plastics, synthetic fibers, rubber, pesticides, and other chemicals. It is a known carcinogen and can cause health problems.
| IUPAC name | Benzene |
| Molecular formula | C6H6 |
| CAS number | 71-43-2 |
| Synonyms | Benzol, Phene, Annulene, Cyclohexatriene, etc. |
| InChI | InChI=1S/C6H6/c1-2-4-6-5-3-1/h1-6H |
Benzene Properties
Benzene molar mass
Benzene has a molar mass of 78.11 g/mol. It is a cyclic organic compound consisting of six carbon atoms and six hydrogen atoms. The molar mass of benzene is calculated by adding the atomic masses of its constituent atoms, which are 12.01 g/mol for carbon and 1.01 g/mol for hydrogen. Benzene is widely used in the chemical industry as a starting material for the production of many important compounds. The molar mass of benzene is important in determining the quantity of the compound needed for reactions and other applications.
Benzene formula
Benzene has the chemical formula C6H6. This formula represents the number and type of atoms present in the molecule. The formula of benzene is important in determining its physical and chemical properties, as well as its reactivity in chemical reactions. The formula of benzene is also used in the naming and identification of the compound in various contexts, such as in chemical literature and regulatory documents.
Benzene boiling point
Benzene has a boiling point of 80.1°C or 176.2°F. This is relatively low compared to other organic compounds with similar molecular weight. The boiling point of C6H6 is due to its intermolecular forces of attraction, specifically van der Waals forces. As the temperature increases, the kinetic energy of the molecules also increases, eventually leading to the breaking of these intermolecular forces and the transition from liquid to gas phase. The low boiling point of C6H6 makes it useful in applications where it is necessary to remove the compound by evaporation.
Benzene melting point
C6H6 has a melting point of 5.5°C or 41.9°F. This is relatively low compared to other aromatic compounds with similar molecular weight. The melting point of C6H6 is due to its molecular structure, which consists of a planar ring of carbon atoms with alternating double bonds. The arrangement of the electrons in the ring gives C6H6 its characteristic stability and makes it resistant to many chemical reactions. The low melting point of C6H6 makes it useful in applications where it is necessary to melt the compound for further processing.
Benzene density g/ml
Benzene has a density of 0.879 g/mL at room temperature (25°C or 77°F). The density of benzene is a measure of the mass per unit volume of the compound. The low density of benzene makes it useful in applications where a low-density solvent is required, such as in the separation of compounds based on their densities.
Benzene molecular weight
Benzene has a molecular weight of 78.11 g/mol. The molecular weight of benzene is the sum of the atomic weights of its constituent atoms. The molecular weight of benzene is important in determining the stoichiometry of reactions and in the determination of the concentration of benzene in solutions.
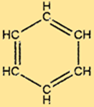
Benzene Structure
C6H6 has a unique structure consisting of a hexagonal ring of carbon atoms, each of which is bonded to two other carbon atoms and one hydrogen atom. The alternating double bonds in the ring give C6H6 its characteristic stability and make it resistant to many chemical reactions. The structure of C6H6 also makes it a useful starting material for the production of many important compounds.
| Appearance | Colorless liquid |
| Specific Gravity | 0.879 g/mL |
| Color | Colorless |
| Odor | Sweet |
| Molar Mass | 78.11 g/mol |
| Density | 0.879 g/mL |
| Melting Point | 5.5°C (41.9°F) |
| Boiling Point | 80.1°C (176.2°F) |
| Flash Point | -11°C (12.2°F) |
| Water Solubility | 1.79 g/L |
| Solubility | Insoluble in water, soluble in organic solvents |
| Vapour Pressure | 12.1 kPa at 20°C |
| Vapour Density | 2.8 (air=1) |
| pKa | 43.2 |
| pH | Neutral |
Benzene Safety and Hazards
C6H6 is a highly flammable and toxic compound that poses significant safety hazards to human health and the environment. It is a known carcinogen and can cause leukemia, aplastic anemia, and other blood disorders in humans. C6H6 exposure can occur through inhalation, skin contact, or ingestion, and can lead to acute and chronic health effects. It is important to handle C6H6 with appropriate safety precautions, including proper ventilation, protective clothing, and respiratory protection. C6H6 should also be stored and disposed of in accordance with local regulations to minimize environmental contamination and risks to public health.
| Hazard Symbols | Skull and crossbones, Flame |
| Safety Description | Highly flammable, Toxic, Carcinogenic, Harmful if swallowed, Irritant |
| UN Ids | UN 1114 (Benzene), UN 1992 (Flammable liquids, toxic) |
| HS Code | 2902.20.00 |
| Hazard Class | 3 (Flammable liquids), 6.1 (Toxic substances), 8 (Corrosive substances) |
| Packing Group | II (Benzene), III (Flammable liquids, toxic) |
| Toxicity | Acute toxicity: Very toxic (LD50: 2.5 g/kg oral, 0.44 mg/L inhalation), Chronic toxicity: Carcinogenic, Mutagenic, Reproductive toxicity |
Benzene Synthesis Methods
Several methods exist for synthesizing benzene, an important industrial chemical used in the production of plastics, rubber, and pharmaceuticals.
One common method is the catalytic reforming of petroleum naphtha, which involves heating the naphtha to high temperatures in the presence of a catalyst, such as platinum or rhenium, to produce a mixture of aromatic hydrocarbons, including benzene.
Another method is the toluene hydrodealkylation, which involves reacting toluene with hydrogen in the presence of a catalyst, such as chromium oxide or molybdenum oxide, to remove the methyl group and form benzene.
Pyrolyzing hydrocarbons, such as coal or petroleum, at high temperatures in the absence of oxygen synthesizes benzene. This process results in the formation of a mixture of aromatic hydrocarbons, including benzene.
Other methods for synthesizing benzene include the reaction of acetylene with itself in the presence of a catalyst, such as copper, to form benzene, as well as the reaction of phenol with formaldehyde in the presence of an acid catalyst, such as sulfuric acid, to produce benzene.
Each of these methods has its own advantages and disadvantages, depending on factors such as cost, efficiency, and environmental impact. The choice of method for synthesizing benzene will depend on the specific application and requirements of the production process.
Benzene Uses
Benzene is a versatile and important industrial chemical with a wide range of applications across various industries. Some of the major uses of benzene are:
- Production of plastics: Used in the production of various types of plastics, including polystyrene, polyurethane, and nylon.
- Production of rubber: Synthetic rubber producers use it in their production. Manufacturers then use this synthetic rubber to create tires, hoses, belts, and other rubber products.
- Production of pharmaceuticals: Used as a starting material for the production of various pharmaceuticals, including antibiotics, antihistamines, and analgesics.
- Solvent: Used as a solvent for a wide range of organic compounds, including fats, oils, resins, and waxes.
- Fuel: Used as a high-octane fuel additive, improving the performance of gasoline engines.
- Extraction of oils: Used in the extraction of oils, such as vegetable oils and essential oils, from plants.
- Adhesives: Used in the production of adhesives, such as rubber cement and contact cement.
- Dyes: Used in the production of various types of dyes, including synthetic dyes used in the textile industry.
Questions:
Q: Is benzene polar?
A: No, benzene is a nonpolar molecule because it has a symmetrical structure and the individual bond polarities cancel out.
Q: What is benzene used for?
A: Benzene is used in the production of various materials, including plastics, rubber, pharmaceuticals, solvents, fuel additives, adhesives, and dyes.
Q: Which compound will conduct electricity when it is dissolved in water? CH4, CuSO4, C6H6, or C6H12O6?
A: CuSO4 will conduct electricity when dissolved in water because it dissociates into ions, allowing for the flow of electric current.
Q: Is C6H6 polar or nonpolar?
A: C6H6, also known as benzene, is a nonpolar molecule because it has a symmetrical structure and the individual bond polarities cancel out.
Q: Is C6H6 soluble in water?
A: No, C6H6 is not soluble in water because it is a nonpolar molecule and does not interact well with polar solvents like water.
