Sodium Carbonate or Soda ash is an inorganic compound with the chemical formula Na2CO3. It is an alkaline salt, white in color and soluble in water. It is used in many industrial and commercial processes, such as in the manufacture of glass, paper, soaps, detergents, and cleaning agents.
| IUPAC Name | Sodium Carbonate |
| Molecular Formula | Na2CO3 |
| CAS Number | 497-19-8 |
| Synonyms | Na2CO3 Anhydrous, Na2CO3 Decahydrate, Washing Soda, Soda Ash |
| InChI | InChI=1S/2Na.CO3/c21-3(2)4;/h2(H,1,2,3,4);/q;;+2/p-2 |
Sodium Carbonate Properties
Sodium Carbonate Molar Mass
The molar mass of sodium carbonate is 106.0 g/mol. It is the sum of the atomic masses of all the atoms in one molecule of sodium carbonate. The molar mass is an important physical property used in many calculations in chemistry and other sciences.
Sodium Carbonate Boiling Point
Sodium carbonate has a boiling point of 1,600°C. This is the temperature at which the vapor pressure of the liquid equals the pressure of the surrounding atmosphere and the liquid boils. The boiling point is a useful property for identifying and separating different compounds.
Sodium Carbonate Melting Point
Na2CO3 has a melting point of 851°C. This is the temperature at which the solid form of the compound changes to a liquid. The melting point is an important property used in the identification and purification of different substances.
Sodium Carbonate Density g/mL
Na2CO3 has a density of 2.54 g/mL. Density is defined as the mass per unit volume of a substance and is a useful property for determining the concentration of a solution.
Sodium Carbonate Molecular Weight
The molecular weight of sodium carbonate is 106 g/mol. This is the sum of the atomic weights of all the atoms in one molecule. The molecular weight is an important property used in many calculations in chemistry and other sciences.
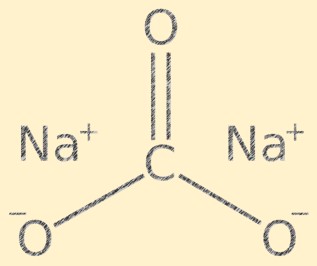
Sodium Carbonate Structure
Na2CO3 has a crystalline structure and is composed of sodium cations (Na+) and carbonate anions (CO3-). The carbonate ion has a trigonal planar shape, with the carbon atom at the center and the oxygen atoms bonded to it at an angle of 120 degrees. The sodium ions and carbonate ions are held together by ionic bonds, giving the compound its characteristic crystal structure.
| Appearance | White crystalline powder or small white crystals |
| Specific Gravity | 2.54 g/cm³ |
| Color | White |
| Odor | Odorless |
| Molar Mass | 106.0 g/mol |
| Density | 2.54 g/mL |
| Melting Point | 851°C |
| Boiling Point | 1,600°C |
| Flash Point | Not applicable |
| Water Solubility | Soluble in water |
| Solubility | Soluble in glycerol, slightly soluble in ethanol |
| Vapor Pressure | Not available |
| Vapor Density | Not available |
| pKa | 10.3 |
| pH | 11.6 (1 M solution) |
Sodium Carbonate Safety and Hazards
Soda ash is a basic compound and can cause skin and eye irritation. Inhaling its dust may cause respiratory irritation. It is also harmful if ingested and can cause digestive upset. Handling should be done with gloves and eye protection, and the substance should be stored in a cool, dry place away from sources of ignition. Avoid ingesting or inhaling the powder and wash thoroughly after handling.
| Hazard Symbols | Xi, N |
| Safety Description | Irritant |
| UN IDs | UN 1824 |
| HS Code | 2836.90.90 |
| Hazard Class | 8 |
| Packing Group | III |
| Toxicity | Oral LD50 (rat) = 915 mg/kg. Inhalation LC50 (rat) = >10 mg/L/4h. May cause skin and eye irritation, respiratory irritation if inhaled. May be harmful if ingested. |
Sodium Carbonate’s Synthesis Methods
Soda ash can be synthesized through two main methods: the Solvay process and the precipitation method. The Solvay process, also known as the ammonia-soda process, involves the reaction of sodium chloride, ammonia, and carbon dioxide to produce soda ash, ammonium chloride, and water. The precipitation method involves the reaction of sodium hydroxide and carbon dioxide to produce soda ash and water. Both methods result in a high purity product that can be used for various applications such as water treatment, soap and detergent manufacturing, and glass production. The Solvay process is the more commonly used method due to its lower production cost and higher production yield.
Sodium Carbonate’s Uses
Industries use soda ash or washing soda, for various purposes. The detergent industry employs it as a water softener and pH adjuster, the glass industry uses it to create flat glass, container glass, and special glass, and the paper industry uses it to neutralize acidic waste streams and adjust pH. In the chemical industry, soda ash is a key ingredient in producing sodium silicates and bicarbonates. It also serves as a food additive and cleaning agent in boilers and water treatment processes in the food industry. Additionally, soda ash plays a role in the production of dyes, enamels, ceramics, and acts as a fire suppressant in fire extinguishers.
