Ammonium chloride or NH4Cl is a white crystalline salt used in various applications, including medicine and fertilizers. It is formed by the reaction of ammonia and hydrochloric acid and has a distinct salty taste.
| IUPAC Name | Ammonium chloride |
| Molecular Formula | NH4Cl |
| CAS Number | 12125-02-9 |
| Synonyms | Sal ammoniac, Salmiac, Nushadir salt, Amchlor, Sal armagnac, Hydrochlorate, Ammonium muriate, Sal armagnac |
| InChI | InChI=1S/ClH.H3N/h1H;1H3 |
NH4Cl molar mass
Ammonium chloride has a molar mass of 53.49 g/mol. Molar mass refers to the mass of one mole of a substance, and it is expressed in grams per mole (g/mol). The molar mass of ammonium chloride is determined by adding the atomic masses of its constituent atoms, which are nitrogen (N), hydrogen (H), and chlorine (Cl). The molecular formula of ammonium chloride is NH4Cl, which means it contains one nitrogen atom, four hydrogen atoms, and one chlorine atom. Therefore, the molar mass of ammonium chloride can be calculated as follows
Molar mass = (1 x atomic mass of N) + (4 x atomic mass of H) + (1 x atomic mass of Cl)
= (1 x 14.01 g/mol) + (4 x 1.01 g/mol) + (1 x 35.45 g/mol)
= 53.49 g/mol
Ammonium chloride boiling point
The boiling point of ammonium chloride is 520 °C (968 °F). The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure applied to it. At this temperature, ammonium chloride will evaporate and turn into its gaseous state. The high boiling point of ammonium chloride is due to its strong ionic bonds between ammonium (NH4+) and chloride (Cl-) ions.
Ammonium chloride melting point
The melting point of ammonium chloride is 338 °C (640 °F). The melting point is the temperature at which a solid turns into a liquid. At this temperature, the ionic bonds between ammonium (NH4+) and chloride (Cl-) ions are broken, and ammonium chloride changes from a solid to a liquid. Ammonium chloride has a relatively low melting point compared to other ionic compounds, which makes it useful in certain applications such as soldering and metalwork.
Ammonium chloride density g/ml
The density of ammonium chloride is 1.527 g/cm³. Density is a measure of the amount of mass per unit volume of a substance. The high density of ammonium chloride is due to its closely packed ionic lattice structure. Ammonium chloride has a higher density than water, which means it will sink in water.
Ammonium chloride molecular weight
The molecular weight of ammonium chloride is 53.49 g/mol. Molecular weight is the sum of the atomic weights of all atoms in a molecule. The molecular weight of ammonium chloride is the same as its molar mass.
Ammonium chloride structure
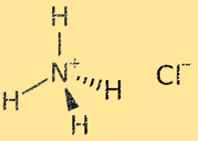
The structure of ammonium chloride is a crystal lattice composed of alternating ammonium (NH4+) and chloride (Cl-) ions. The ammonium ion is a polyatomic ion with a positive charge (+1), consisting of one nitrogen atom and four hydrogen atoms. The chloride ion is a monatomic ion with a negative charge (-1), consisting of one chlorine atom. The crystal lattice structure of ammonium chloride is stabilized by strong ionic bonds between ammonium and chloride ions, resulting in a highly stable and brittle solid.
Ammonium chloride formula
The formula of ammonium chloride is NH4Cl. It is composed of one ammonium ion (NH4+) and one chloride ion (Cl-). The formula represents the ratio of atoms in a molecule, and it shows that for every ammonium ion, there is one chloride ion. The formula of ammonium chloride is used to determine the molar mass, density, and other properties of the compound.
| Appearance | White crystalline solid |
| Specific Gravity | 1.527 g/cm³ |
| Color | Colorless to white |
| Odor | Odorless |
| Molar Mass | 53.49 g/mol |
| Density | 1.527 g/cm³ |
| Melting Point | 338 °C (640 °F) |
| Boiling Point | 520 °C (968 °F) |
| Flash Point | Not applicable |
| Water Solubility | 29.7 g/100 mL (25 °C) |
| Solubility | Soluble in ethanol and acetone |
| Vapour Pressure | Not applicable |
| Vapour Density | Not applicable |
| PKa | 9.25 |
| pH | 4.6 (5% solution) |
Ammonium Chloride Safety and Hazards
Ammonium chloride (NH4Cl) is generally considered to be safe for normal use. However, it can be harmful if ingested or inhaled in large quantities. Ingesting NH4Cl can cause irritation of the mouth and throat, nausea, vomiting, and diarrhea. Inhaling NH4Cl can cause irritation of the respiratory system, coughing, and shortness of breath. NH4Cl should be handled with care, as it can cause skin irritation and eye irritation. It is also important to avoid exposure to NH4Cl dust or fumes, which can be irritating to the eyes, nose, and throat. Proper personal protective equipment, such as gloves and goggles, should be worn when handling NH4Cl.
| Hazard Symbols | Xi |
| Safety Description | S22, S24/25, S36/37/39 |
| UN IDs | UN 3077 |
| HS Code | 2827.10.00 |
| Hazard Class | 9 – Miscellaneous dangerous goods |
| Packing Group | III |
| Toxicity | Low toxicity, but can be harmful if ingested or inhaled in large quantities. |
Ammonium Chloride Synthesis Methods
One can synthesize ammonium chloride (NH4Cl) through various methods.
- One common method involves reacting ammonia gas with hydrochloric acid in water. This exothermic reaction produces NH4Cl as a white crystalline solid. It is important to add the hydrochloric acid to the water slowly while stirring to avoid splashing due to the heat generated.
- Another method of producing NH4Cl is as a by-product of various industrial processes such as the production of sodium carbonate or the Solvay process.
- Another way to synthesize NH4Cl is by reacting ammonia with sodium chloride. This reaction produces both NH4Cl and sodium hydroxide as products. This reaction is exothermic and requires careful control to prevent unwanted by-products formation.
Ammonium Chloride Uses
Ammonium chloride (NH4Cl) has a variety of uses in different industries.
- NH4Cl serves as a fertilizer in agriculture, providing essential nutrients to crops like nitrogen and chloride, thereby improving yield and quality.
- Ammonium chloride facilitates proper functioning of dry cell batteries by conducting electricity as an electrolyte.
- The textile industry employs NH4Cl as a dyeing and printing agent, which helps to fix dyes to fabrics and improve their color fastness.
- As a flux in metalwork and soldering, NH4Cl removes oxide coatings from metals, allowing for easier soldering.
- The chemical has applications in food, pharmaceuticals, and cosmetics as a flavoring agent, expectorant in cough medicines, and ingredient in personal care products.
Overall, the unique properties of NH4Cl make it a versatile chemical with many important applications across different industries.
Questions:
Q: Is ammonium chloride sublime?
A: Yes, ammonium chloride is known to sublime, meaning it can transition directly from a solid to a gas phase without melting in between.
Q: Is NH4Cl acid or base?
A: Ammonium chloride is an acidic salt. When dissolved in water, it can undergo hydrolysis to produce an acidic solution. This is due to the ammonium ion acting as a weak acid and donating a proton (H+) to the water molecules.
Q: What is the formula of ammonium chloride?
A: The formula of ammonium chloride is NH4Cl. It is composed of one ammonium ion (NH4+) and one chloride ion (Cl-) held together by ionic bonds.
Q: Does ammonium chloride dissolve in water?
A: Yes, ammonium chloride is highly soluble in water. At room temperature, approximately 29.7 grams of ammonium chloride can dissolve in 100 milliliters of water.
Q: How to separate ammonium chloride and sodium chloride?
A: One way to separate ammonium chloride and sodium chloride is through sublimation. This can be achieved by heating the mixture, causing the ammonium chloride to sublime while the sodium chloride remains behind as a solid. The sublimed ammonium chloride can then be collected and condensed back into a solid form. Another method involves dissolving the mixture in water and then selectively precipitating out one of the salts using a specific reagent or pH adjustment.
