The carbonate ion, CO32-, is a negatively charged ion consisting of one carbon atom, three oxygen atoms, and an overall charge of -2. It is commonly found in minerals such as calcite and dolomite, as well as in aqueous solutions in the form of bicarbonate. It plays a crucial role in controlling pH levels in the ocean and other bodies of water.
| IUPAC Name | Carbonate Ion |
| Molecular Formula | CO3 2- |
| CAS number | 497-19-8 |
| Synonyms | Carbonic acid, Carbon trioxide |
| InChI | InChI=1S/CH2O3.2K/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2 |
Carbonate Ion Properties
Carbonate Formula
The carbonate ion formula is represented as CO3^2-, where C stands for carbon, O stands for oxygen, and the 2- symbol represents the negative charge of the ion. This formula indicates that there are three oxygen atoms and one carbon atom in the carbonate ion, giving it its characteristic molecular structure.
Carbonate CO3 Charge
The carbonate ion, CO3^2-, has a negative 2 charge due to the presence of three oxygen atoms and one carbon atom in its chemical formula. The negative charge arises from the presence of extra electrons within the carbonate ion, making it a negatively charged ion.
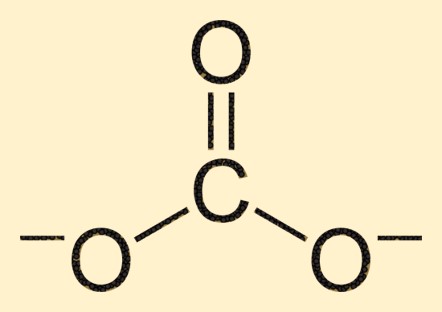
Carbonate Structure
The carbonate ion is composed of a carbon atom at its center, surrounded by three oxygen atoms in a trigonal planar arrangement. The carbon atom forms a double bond with one oxygen atom, while the other two oxygen atoms are bonded to the carbon atom through single bonds. This structure gives the CO3 2-ion its characteristic shape and properties.
Carbonate Ion Molecular Weight
The molecular weight of the carbonate ion is approximately 60.01 g/mol, which is calculated based on the atomic weights of its constituent elements (carbon, oxygen). The molecular weight of the carbonic acid is important for determining the amount of carbonic acid in a sample, as well as for determining its physical and chemical properties.
| Appearance | Solid, white powder |
| Specific Gravity | 2.5 |
| Color | White |
| Odor | None |
| Molar Mass | 100.09 g/mol |
| Density | 2.83 g/cm3 |
| Melting Point | 825°C |
| Boiling Point | 1484°C |
| Flash Point | Not applicable |
| Water Solubility | Soluble in water |
| Solubility | Soluble in acids |
| Vapour Pressure | Not applicable |
| Vapour Density | Not applicable |
| PKa | 10.33 |
| PH | 8.6 |
Carbonate Ion Safety and Hazards
Carbonate ion, CO32-, is widely used in various industries, but it must be handled with caution. Inhaling high levels of carbonate dust can cause respiratory problems, while ingesting it in large amounts can lead to digestive issues. Skin contact can cause irritation and skin damage. It is also flammable and can react with other chemicals to produce hazardous gases. It is important to follow safety measures, such as wearing personal protective equipment and storing carbonate ion in proper containers, to minimize the risk of accidents.
| Hazard Symbol | None |
| Safety Description | It is not considered hazardous and does not have any specific hazard symbols associated with it. |
| UN ID | N/A |
| HS Code | N/A |
| Hazard Class | N/A |
| Packing Group | N/A |
| Toxicity | Non-toxic |
Carbonate Ion Synthesis Methods
Carbonate ions (CO3 2-) can be synthesised through a variety of different methods. The most common method of producing CO3 2- ions is through the reaction of carbon dioxide and water. This reaction produces a solution of carbonic acid, which can then be neutralised with an alkali, such as sodium hydroxide, to produce carbonic acid. Carbonic acid can also be produced through electrolysis of a salt solution, where the carbonate ions are formed at the cathode. These ions can also be produced in a laboratory setting through a chemical reaction between an acid and a carbonate salt.
Carbonate Ion Uses
Industries widely use carbonic acid for various purposes. Baking powder manufacturers, antacid producers, and beverage companies commonly incorporate carbonic acid in their products. The water treatment industry controls pH levels in drinking water and swimming pools using carbonate ions. The building industry uses CO3 2- ions as a main component of cement to make concrete. Farmers add carbonate ions to soil to improve its alkalinity and enhance crop growth. The chemical industry uses carbonate ions as a catalyst and a reactant in various chemical reactions. The food industry preserves food by incorporating carbonate ions. Due to its versatility, carbonate ions play an essential role in many everyday products and industrial processes.
