Aluminium chloride or AlCl3 is a chemical compound used in various industrial processes. It acts as a Lewis acid, catalyzing reactions in organic synthesis and polymerization. It also has antiperspirant properties in personal care products.
| IUPAC Name | Aluminum trichloride |
| Molecular Formula | AlCl3 |
| CAS Number | 7446-70-0 |
| Synonyms | Aluminum trichloride, Aluminum(III) chloride, Aluminum(III) chloride, Aluminum chloride, Trichloroaluminum |
| InChI | InChI=1S/3ClH.Al/h3*1H;/q;;;+3/p-3 |
Aluminium chloride formula
The chemical formula of aluminium chloride is AlCl3. This formula represents the number of atoms of each element in the molecule. The formula of aluminium chloride is important because it is used to identify the substance and to calculate its properties. The formula is also used in chemical equations to represent the reactants and products of a chemical reaction.
Aluminium chloride molar mass
Aluminium chloride has a molar mass of 133.34 g/mol. This value is calculated by adding the atomic mass of one aluminium atom and three chlorine atoms. The molecular formula of aluminium chloride is AlCl3, which means that it contains one aluminium atom and three chlorine atoms. The molar mass is an important property of aluminium chloride because it is used in many chemical reactions and industrial processes. The molar mass is also used to calculate the amount of a substance present in a given sample.
Aluminium chloride boiling point
The boiling point of aluminium chloride is 180.8 °C (357.4 °F). This value is the temperature at which the liquid form of aluminium chloride changes into its gaseous state. The boiling point of aluminium chloride is relatively high, which makes it useful in many industrial processes that require high temperatures. For example, aluminium chloride is used as a catalyst in the manufacture of polypropylene, a thermoplastic polymer.
Aluminium chloride melting point
The melting point of aluminium chloride is 194 °C (381.2 °F). This value is the temperature at which the solid form of aluminium chloride changes into its liquid state. The melting point of aluminium chloride is relatively low, which means that it can easily be melted and used in various chemical reactions and industrial processes. For example, aluminium chloride is used as a coagulant in the water treatment industry.
Aluminium chloride density g/ml
The density of aluminium chloride is 2.44 g/cm3. This value is the mass of aluminium chloride per unit volume. The density of aluminium chloride is important because it determines the mass of a given volume of the substance. The density of aluminium chloride is also used to calculate the concentration of the substance in a solution.
Aluminium chloride structure
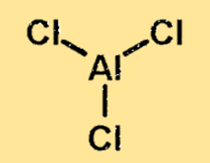
The structure of aluminium chloride is a covalently bonded molecule with one aluminium atom and three chlorine atoms. The aluminium atom is located in the center of the molecule, with the three chlorine atoms surrounding it. The structure of aluminium chloride is important because it determines the physical and chemical properties of the substance.
Aluminium chloride molecular weight
The molecular weight of aluminium chloride is 133.34 g/mol. This value is the sum of the atomic weights of the atoms in the molecule. The molecular weight of aluminium chloride is important because it is used to calculate the amount of a substance present in a given sample. This value is also used to determine the molar concentration of a solution.
| Appearance | White to yellow solid or powder |
| Specific Gravity | 2.44 g/cm3 |
| Color | Colorless to pale yellow |
| Odor | Odorless |
| Molar Mass | 133.34 g/mol |
| Density | 2.44 g/cm3 |
| Melting Point | 194 °C (381.2 °F) |
| Boiling Point | 180.8 °C (357.4 °F) |
| Flash Point | Not applicable |
| Water Solubility | Highly soluble in water |
| Solubility | Soluble in ethanol, methanol, and ether |
| Vapour Pressure | 2.67 kPa at 25 °C |
| Vapour Density | 4.45 (air = 1) |
| pKa | -6 |
| pH | 2.0 (10 g/L, H2O) |
Aluminum Chloride Safety and Hazards
Aluminum chloride poses several safety and health hazards. It is corrosive and can cause severe skin and eye irritation, as well as respiratory and digestive tract irritation if ingested or inhaled. It can also cause burns if it comes into contact with the skin. Aluminum chloride is a strong oxidizing agent and can react violently with water, releasing hydrogen chloride gas. It is also incompatible with many organic compounds, such as alcohols and amines, and can react with them to produce hazardous gases. Proper protective equipment, including gloves, goggles, and a respirator, should be worn when handling aluminum chloride.
| Hazard Symbols | Corrosive, Harmful |
| Safety Description | Avoid contact with skin and eyes. Wear protective gloves and eye/face protection. In case of contact, rinse thoroughly with water and seek medical attention. Do not ingest or inhale. Use in a well-ventilated area. Store in a cool, dry place away from incompatible materials. |
| UN IDs | UN 1726 |
| HS Code | 2827.32.00 |
| Hazard Class | 8 |
| Packing Group | III |
| Toxicity | Toxic if ingested or inhaled. Causes severe skin and eye irritation. May cause respiratory and digestive tract irritation. Can react violently with water, releasing hydrogen chloride gas. Incompatible with many organic compounds, such as alcohols and amines, and can react with them to produce hazardous gases. |
Aluminum Chloride Synthesis Methods
One can synthesize aluminum chloride through various methods such as direct synthesis, hydrolysis, and the reaction of aluminum with hydrogen chloride.
- Direct synthesis involves the reaction of aluminum with chlorine gas at high temperatures, typically around 700-800 °C. The reaction of aluminum with hydrogen chloride generates aluminum chloride in the gas phase, which one can condense to a liquid form.
- Hydrolysis involves the reaction of aluminum metal with hydrochloric acid or water, which produces hydrogen gas and aluminum chloride. The hydrolysis reaction is typically exothermic and can be dangerous if not conducted properly.
- The reaction of aluminum with hydrogen chloride gas is another method of synthesizing aluminum chloride. This reaction takes place at room temperature and produces hydrogen gas and aluminum chloride.
- Another common method of synthesizing aluminum chloride is by the reaction of aluminum oxide with hydrochloric acid. This reaction produces aluminum chloride and water as products.
- The reaction of aluminum with a mixture of chlorine gas and carbon monoxide is another method to synthesize aluminum chloride. This reaction takes place at high temperatures and produces aluminum chloride and carbon dioxide.
Overall, the synthesis of aluminum chloride requires careful handling and control of reaction conditions to ensure a safe and effective process.
Aluminum Chloride Uses
Aluminum chloride has numerous uses in various industries due to its unique properties.
- Organic chemistry reactions, including the production of polymers, pharmaceuticals, and fragrances, commonly use aluminum chloride as a catalyst.
- The treatment of drinking water and wastewater to remove suspended solids often involves the use of aluminum chloride as a coagulant.
- The production of aluminum metal and alloys benefits from the use of aluminum chloride as a flux, which helps remove impurities and improve the casting process.
- In the petrochemical industry, aluminum chloride serves as a catalyst in the production of gasoline and other hydrocarbons. Additionally, it is used as a deodorant and antiperspirant in personal care products.
- Some over-the-counter medications use aluminum chloride, which has astringent properties, to treat minor skin irritations and stop bleeding. Aluminum chloride is also used to treat hyperhidrosis, a condition characterized by excessive sweating.
- The textile industry relies on aluminum chloride as a mordant to help fix dyes to fabrics, and it is also used in the production of synthetic rubber.
Overall, aluminum chloride finds diverse and important uses in many industries, highlighting its significance in the modern world.
Questions:
Q: Which best compares 1 mol of sodium chloride to 1 mol of aluminum chloride?
A: 1 mol of sodium chloride (NaCl) and 1 mol of aluminum chloride (AlCl3) both contain 1 mol of chloride ions, but aluminum chloride contains three times as many cations as sodium chloride.
Q: Is aluminum chloride aqueous?
A: Aluminum chloride can exist in both solid and aqueous forms. When dissolved in water, it forms a highly acidic solution due to the hydrolysis of the aluminum cation.
Q: What is the correct formula for aluminum chloride?
A: The correct formula for aluminum chloride is AlCl3. This indicates that each molecule of aluminum chloride contains one aluminum cation (Al3+) and three chloride anions (Cl-).
Q: Is aluminum chloride safe?
A: Aluminum chloride can be hazardous if not handled properly. It can cause skin and eye irritation, respiratory and digestive tract irritation, and may be toxic if ingested or inhaled. It should be stored and handled in a well-ventilated area and with appropriate protective gear.
Q: How many chloride ions are in 1.50 mol of aluminum chloride?
A: Since the formula for aluminum chloride is AlCl3, each molecule contains three chloride ions. Therefore, 1.50 mol of aluminum chloride contains 1.50 x 3 = 4.50 mol of chloride ions.
