Acetate ion (C2H3O2-) is an anion composed of a carbon atom, two hydrogen atoms and an oxygen atom, widely found in organic chemistry and biochemistry.
| IUPAC Name | Ethanoate |
| Molecular Formula | C2H3O2- |
| CAS Number | 127-09-3 |
| Synonyms | Acetic acid anion; Ethanoic acid anion; Acetate anion |
| InChI | InChI=1S/C2H4O2/c1-2(3)4/h1H3,(H,3,4)/p-1 |
Acetate Charge
Acetate ion is an anion, which means that it carries a negative charge. The acetate ion is derived from acetic acid, which is a weak organic acid that dissociates in water to form hydrogen ions (H+) and acetate ions (C2H3O2-). The acetate ion has a charge of -1, and it is a common ion in biological systems and in many chemical reactions.
Acetate Formula
The acetate ion has the chemical formula C2H3O2-. It is a negatively charged ion composed of two carbon atoms, three hydrogen atoms, and two oxygen atoms. The acetate ion is the conjugate base of acetic acid, which has the chemical formula CH3COOH. The acetate ion forms salts with positively charged ions such as sodium (Na+) and calcium (Ca2+) to produce sodium acetate (CH3COO-Na+) and calcium acetate (CH3COO-Ca2+) respectively.
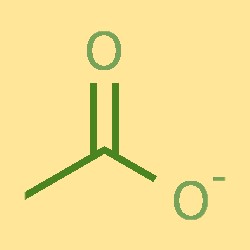
Acetate Ion Structure
The acetate ion consists of a carbon atom, two hydrogen atoms, and an oxygen atom. The carbon atom is bonded to one oxygen atom and two hydrogen atoms, forming a C-O-H structure. The oxygen atom is also bonded to a negatively charged ion. The acetate ion has a trigonal planar shape, with bond angles of approximately 120°.
Acetate Ion Molar Mass
The molar mass of the acetate ion (C2H3O2) is 59.04 g/mol. It is calculated by adding up the atomic weights of all the atoms in the ion. The molar mass is important in determining the amount of substance in a sample.
Acetate Ion Boiling Point
The boiling point of the acetate ion is not applicable as it is a negatively charged ion and does not exist as a standalone molecule. However, the boiling point of acetic acid, which contains the acetate ion, is 118.1°C.
Acetate Ion Molecular Weight
The molecular weight of the acetate ion (C2H3O2) is 59.04 g/mol, which is the sum of the atomic weights of all the atoms in the ion. The molecular weight is useful in determining the amount of substance in a sample.
| Appearance | Not applicable (ion) |
| Specific Gravity | Not applicable (ion) |
| Color | Not applicable (ion) |
| Odor | Not applicable (ion) |
| Molar Mass | 59.04 g/mol |
| Density | Not applicable (ion) |
| Melting Point | Not applicable (ion) |
| Boiling Point | Not applicable (ion) |
| Flash Point | Not applicable (ion) |
| Water Solubility | Soluble in water |
| Solubility | Soluble in water and polar solvents |
| Vapor Pressure | Not applicable (ion) |
| Vapor Density | Not applicable (ion) |
| pKa | 4.76 |
| pH | Acidic |
Acetate Ion Safety and Hazards
Acetate ion is generally considered safe as it is a naturally occurring ion found in many food and beverage products. However, concentrated solutions of acetic acid, which contains the acetate ion, can be harmful if ingested, inhaled or come into contact with skin and eyes, causing irritation or chemical burns. Proper protective equipment and ventilation should be used when handling concentrated solutions of acetic acid.
| Hazard Symbols | Irritant |
| Safety Description | S2 – Keep out of reach of children. S26 – In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S37 – Wear suitable gloves. S60 – This material and its container must be disposed of as hazardous waste. |
| UN IDs | UN2790 |
| HS Code | 2915.90.90 |
| Hazard Class | 8 |
| Packing Group | III |
| Toxicity | Acetate ion is considered low toxicity when ingested, but concentrated solutions of acetic acid (which contains acetate ion) can be harmful if ingested, inhaled or come into contact with skin and eyes. |
Acetate Ion Synthesis Methods
The synthesis of acetate ion typically involves the formation of acetic acid, which is the parent compound that contains the acetate ion. One common method for synthesizing acetic acid involves the oxidation of ethanol using an oxidizing agent such as chromic acid. Another method involves the carbonylation of methanol using carbon monoxide and a catalyst. Acetic acid can also be produced biologically through the fermentation of carbohydrates by acetogenic bacteria. The acetate ion can also be obtained through the dissociation of acetic acid in a solution, which liberates the acetate ion and hydrogen ions. In some cases, the acetate ion can also be obtained through the reaction of an acetic ester with a strong base.
Acetate Ion Uses
Acetate ion (C2H3O2), in the form of acetic acid, serves various purposes across industries. The food industry uses acetic acid as a condiment and preservative due to its sour taste and antimicrobial properties. The textile industry employs acetic acid as a component in fabric softeners and as a dye fixative. In the chemical industry, acetic acid functions as a feedstock for the production of other chemicals such as vinyl acetate, used in adhesives and coatings. The pharmaceutical industry utilizes acetate ions as an ingredient in medications and as a buffer in medical treatments. Additionally, cellulose acetate, a type of plastic, produce using acetic acid and finds application in film and optical fibers.
